Precision antibodies for patients who need better options
We discover medicines developed for obesity and cardiometabolic disease. Integrating, speed, precision, and developability, our AI-assisted platform designs antibodies for hard-to-drug targets.
Learn More

Addressing today’s treatment gaps

We aim to overcome the shortcomings of current approved therapies. Our antibodies are designed to selectively target fat, preserve muscle, decrease dosing frequency, and promote sustained weight loss.
Explore our pipeline
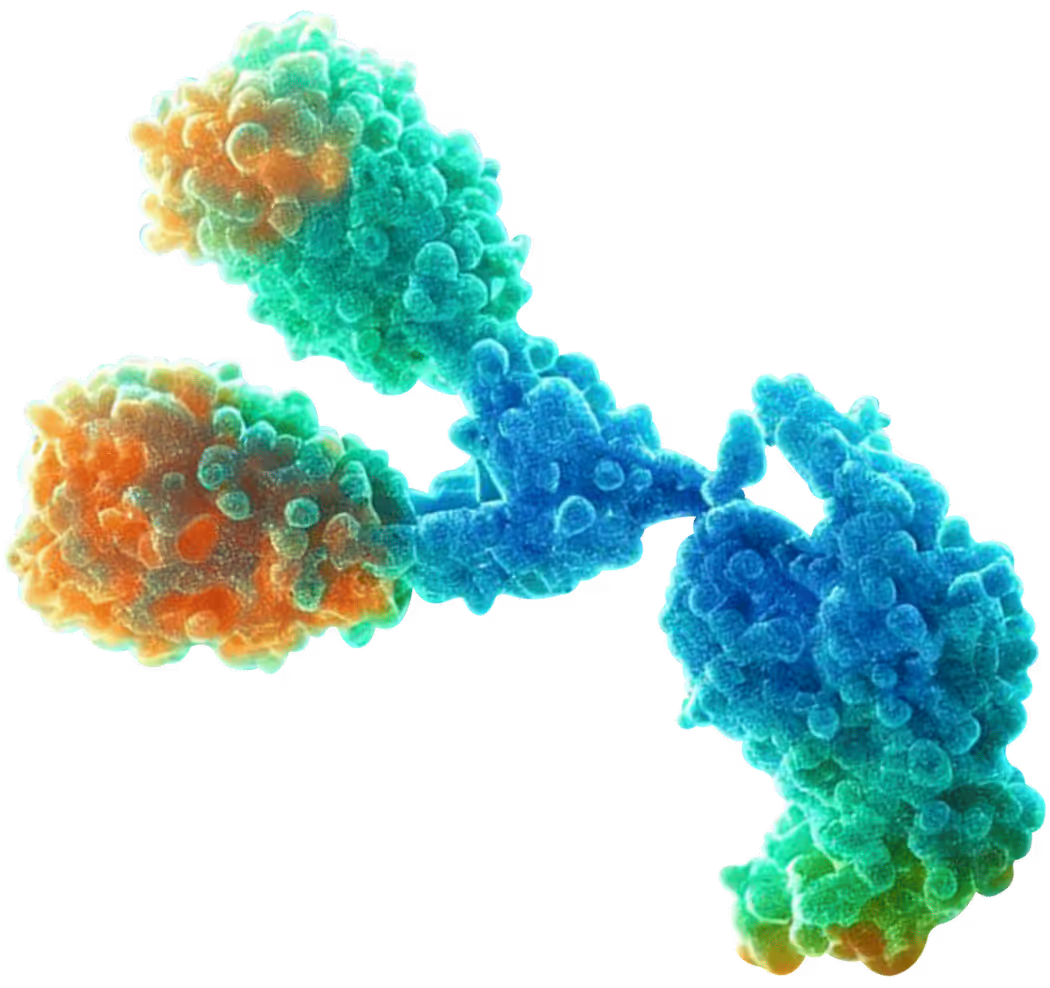
The engine behind our medicines
We build medicines by tightly integrating AI with experimental biology—enabling us to create antibodies against targets that were once considered undruggable. With our Epitope Engineering for pinpoint precision and single-shot StableHu™ Antibody Optimization for speed and developability, our platform delivers therapies engineered for real-world patient impact.
Explore our platform

Built by a team who cares

Martin Brenner, DVM, Ph.D.
Chief Executive Officer and Chief Scientific Officer

Felipe Duran
Chief Financial Officer

Marc Banjak
Chief Legal Officer
Strategic partnerships for greater impact
We collaborate with partners to advance the development of innovative biologics across obesity and other therapeutic areas like immunology and infectious disease.
partner with us

Join a team that’s building the future of medicine
At iBio, we are driven by science and a shared mission to develop innovative biologics that improve patient outcomes. Join our team and drive innovations that matter—working in a collaborative, dynamic environment where your ideas can transform the future of healthcare.
join our team
