Discovery Platform
A Powerful Tech Stack for Validating Difficult Targets
Discovering, engineering, and developing new antibodies with our machine learning platform
License our discovery platform or collaborate with us to advance our high-value pipeline assets


Epitope-Steering and Antibody Optimization for Accelerating Cancer Immunotherapies


From precision antibody identification and optimization to tailored bispecifics and a cutting-edge masking technology


An antibody masking technology for delivering on-epitope, on-tissue clinical candidates with enhanced safety and developability
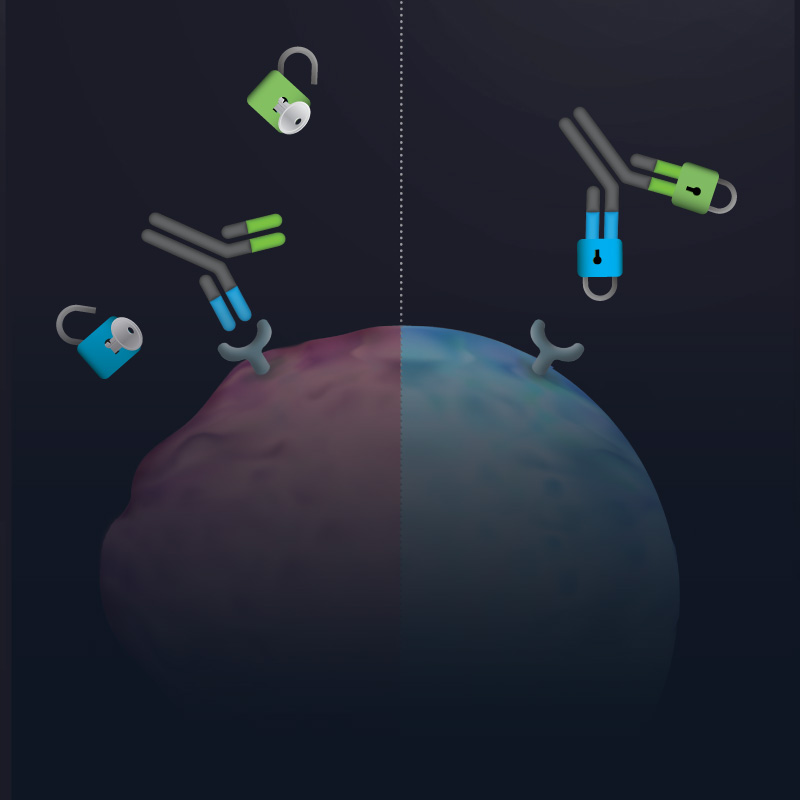
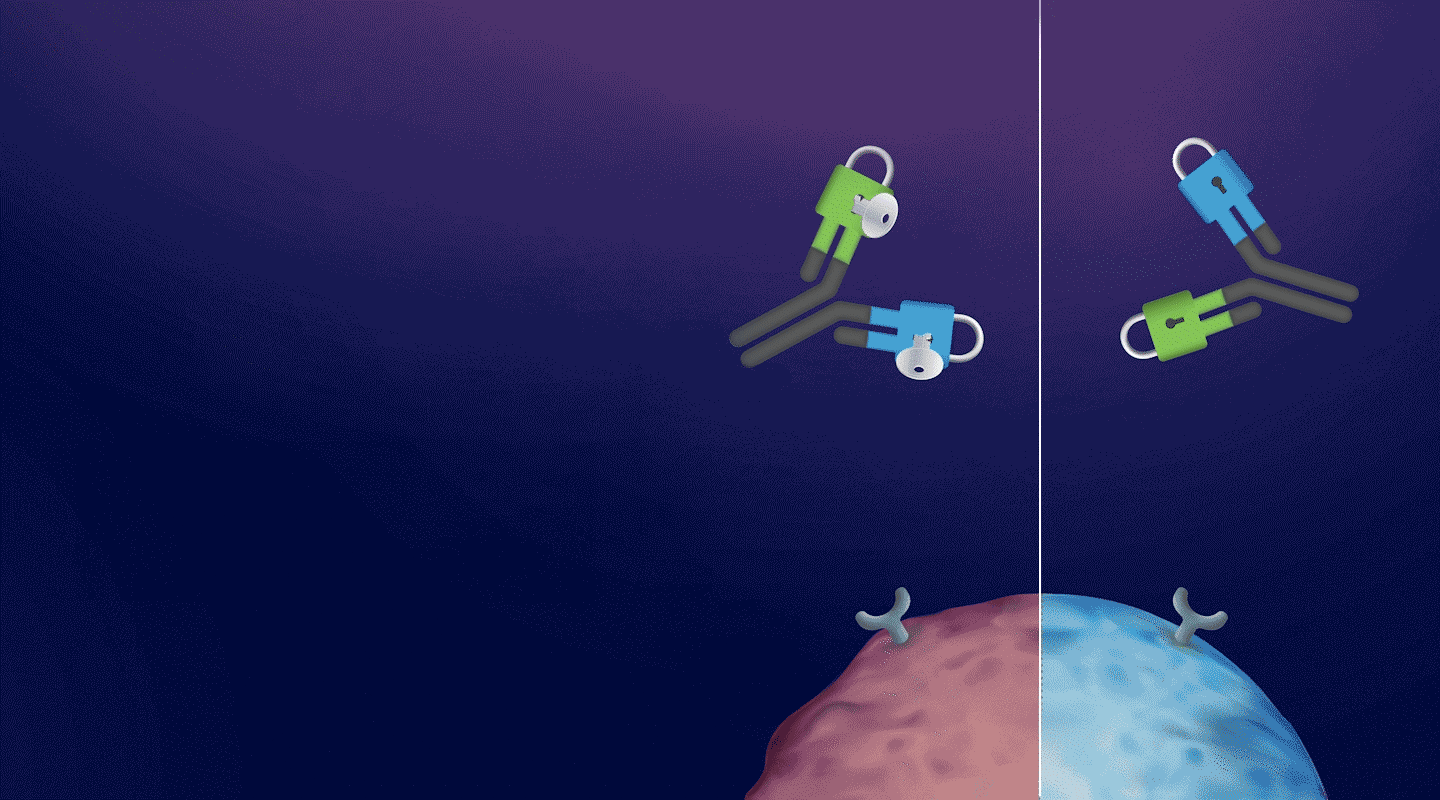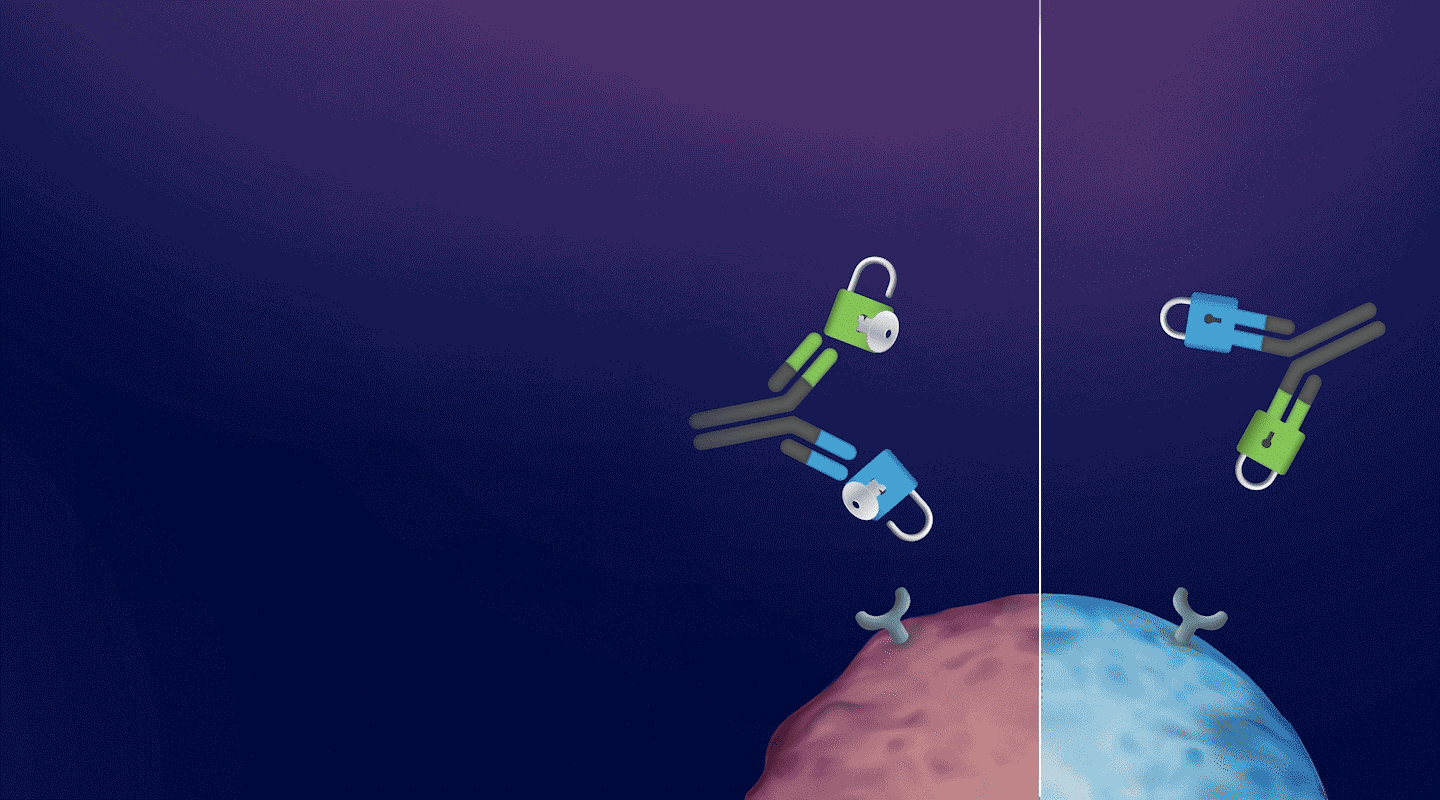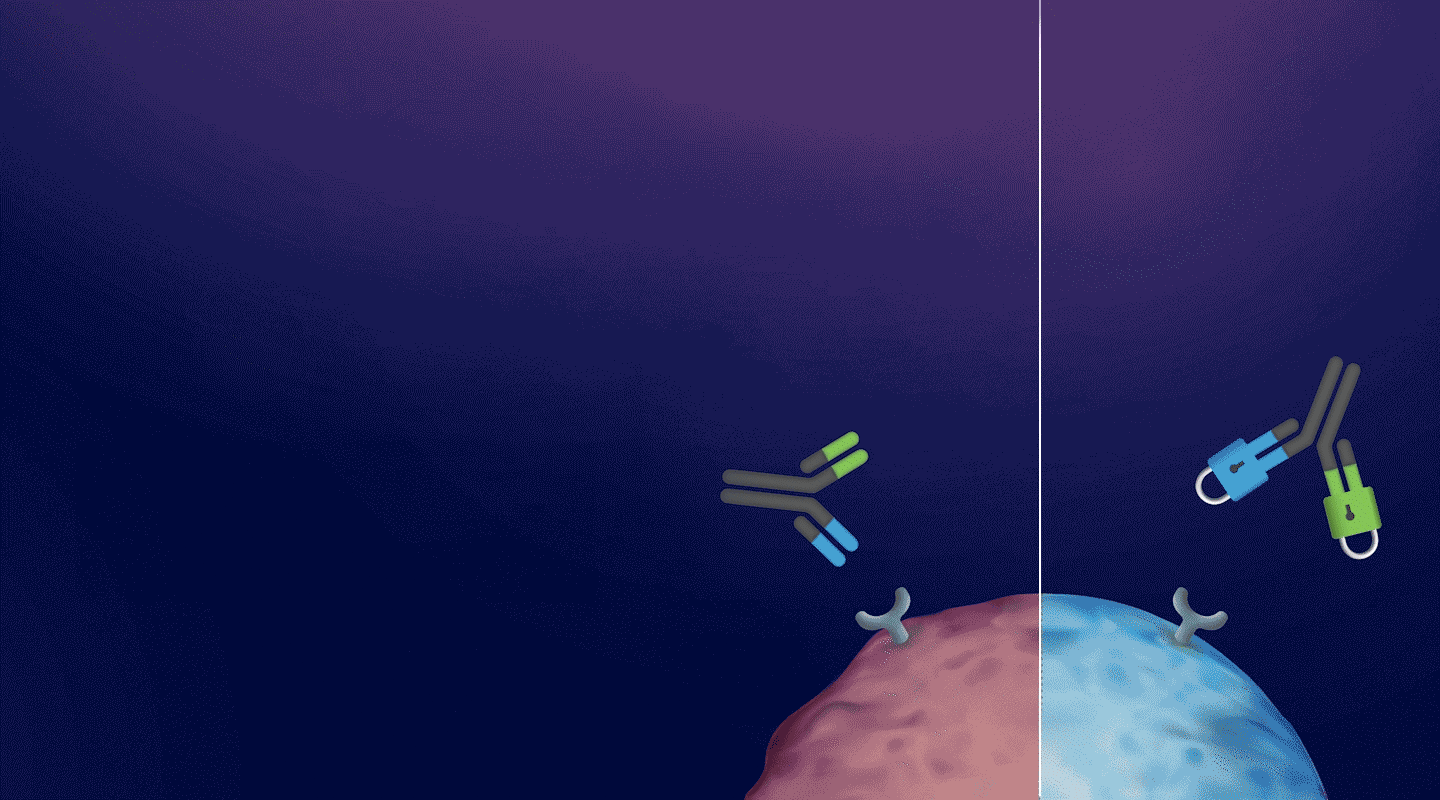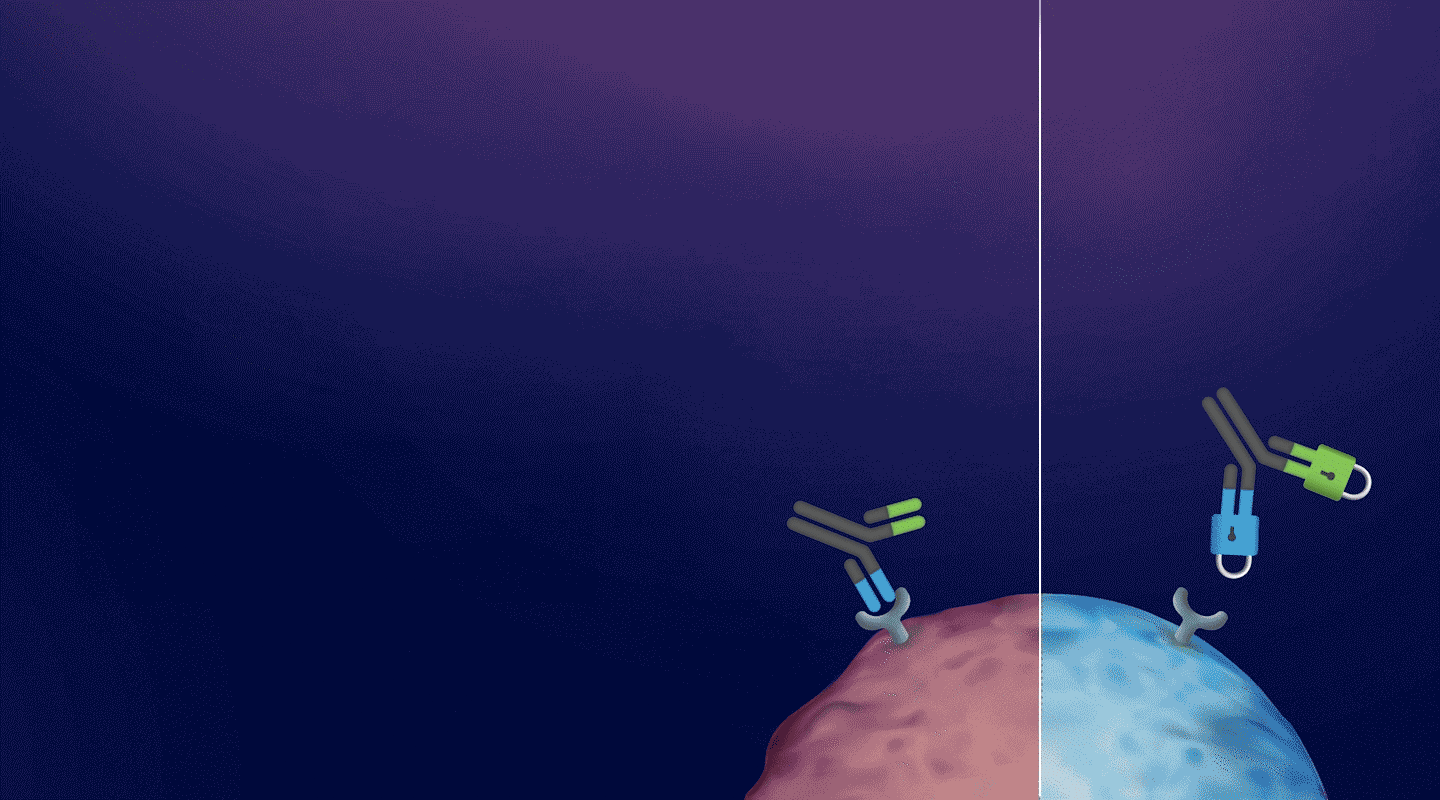
a CD3-BASED T-CELL ENGAGER Platform
RAPIDLY ADVANCING HARD-TO-ENGINEER ANTIBODIES

Learn how we are deploying our AI Drug Discovery Platform to develop a robust immuno-oncology pipeline

Meet the team driving iBio’s powerful tech stack for drug discovery
For Investor inquiries, please visit our Investor Site